São Paulo – Brazilian health regulator Anvisa approved emergency use of CoronaVac and the Oxford vaccine in Brazil. The first is produced by São Paulo’s Butantan medical center that is partnered with China’s Sinovac, while the latter was developed by AstraZeneca and the Oxford University with federally funded Fiocruz institute.
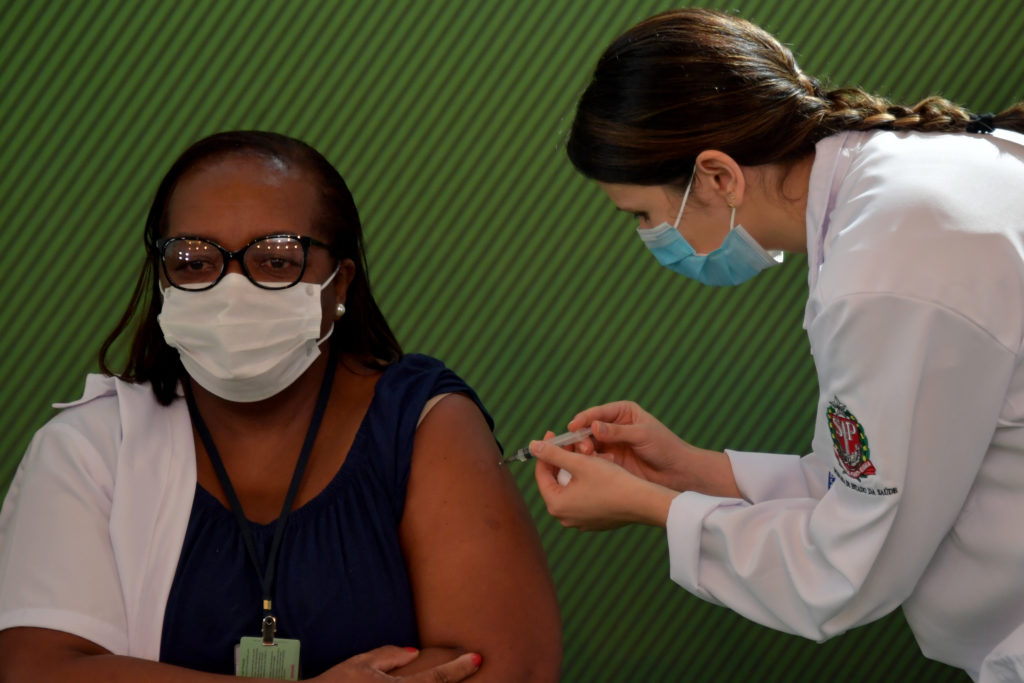
The decision was disclosed on Sunday (17) and, minutes later, Monica Calazans (pictured above), a nurse in São Paulo’s Emílio Ribas infectology hospital, became the first person to be inoculated in the country, receiving the Butantan’s vaccine, the Federal Nursing Service Council reported.
As the emergency use of two vaccines was approved, the federal government decided to kick off the immunization program. Agência Brasil reported that Health minister Eduardo Pazuello said on Monday (18) the novel coronavirus vaccination would start in today. He said Brazilian Air Force planes would start distributing the vaccines to states by 2pm (BRT) and the first vaccines would be given by 5 pm (BRT).
Agência Brasil reported, too, that Pazuello participated with governors in a symbolical delivery of 4.6 million doses of CoronaVac in the Guarulhos Airport’s Logistics Center in São Paulo. The vaccines will be transported by air to the Federal District and capitals of ten states: Acre, Amapá, Amazonas, Ceará, Goiás, Mato Grosso do Sul, Piauí, Rondônia, Roraima, and Santa Catarina. Vaccines are also expected to be distributed by road.
The minister said Butantan will receive a letter asking for it to fast-track its request to produce other 2 million CoronaVac doses. The documents are to be analyzed by March 31.
The minister stated the first people to receive the vaccine doses will be part of the priority groups: frontline health workers, the elderly, and indigenous communities. Pazuello stressed the use of face masks and hand sanitizers must continue. “The vaccine doesn’t mean the end of protective measures,” he said.
Translated by Guilherme Miranda